Photosensitivity reaction, a new drug safety signal for midostaurin identified based on the safety evidence from French pharmacovigilance database, the Base Nationale de Pharmacovigilance (BNPV) and from the global drug safety database, VigiBase which is maintained by WHO-UMC (Uppsala Monitoring Center).
Midostaurin is a multiple tyrosine kinase inhibitor (including FLT3 and KIT kinases) which inhibits mast cell proliferation, survival, and histamine release. This is indicated as monotherapy for the treatment of adult patients with aggressive systemic mastocytosis, systemic mastocytosis with associated haematological neoplasm or mast cell leukaemia. It is also used in combination with daunorubicin and cytarabine and high dose cytarabine consolidation chemotherapy, as well as for patients in complete response, followed by midostaurin as monotherapy for adult patients newly diagnosed with acute myeloid leukaemia (AML) positive for the tyrosine kinase protein ‘fms-like tyrosine kinase 3’ (FLT3) mutation.
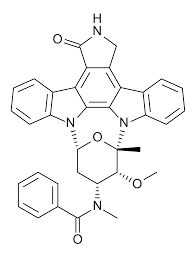
Midostaurin molecular structure
Photosensitivity reactions are the immune system reactions triggered by sunlight which are usually diagnosed by physician evaluation. Some of photosensitivity reactions included itchy eruptions, areas of redness and inflammation on patches of sun-exposed skin which typically resolve without treatment. It is often considered that people with low levels of melanin, white skin and rarely tan are more sensitive to photosensitivity reaction relative to people with darker skin types who tan more easily. Drug-induced photosensitivity reactions occur as a result of the combined effects of a drug and sunlight in susceptible patients and has the potential to increase the incidence of skin cancer.
It is indicated that midostaurin can cause photosensitivity reactions while interacting with sunlight in certain patients based on the case reports present in the French pharmacovigilance database, the BNPV and VigiBase.
Case Reports in French National PV Database:
Total 8 case reports were identified which are serious. (Of 8 cases, two cases were also reported/databased in VigiBase).
Temporal relationship: From 10 days to 1 year for onset of reactions in 8 patients.
Dechallenge: Positive dechallenge reported in one patient.
Possible confounding factors: Concurrent medication like valsartan which are able to cause photosensitivity and patients with history of photosensitive reactions.
Among the reported cases, one case is well reported (Index case) which is reported in 2019.
“Index case that led to contraindication of midostaurin: A patient who had been treated with midostaurin for over 2 months when, after having taken a walk outside in sunny spring weather, experienced a severe photosensitivity reaction. This led to withdrawal of the drug after having had a photosensitivity test performed in hospital where a minimal dose of the drug was given, and UVA-light was applied. A very strong reaction followed, the drug was permanently withdrawn, and the treating physician issued a contraindication for the drug for this patient”.
Global Case Reports:
In VigiBase, total 4 case reports were found with photosensitivity reactions associated with midostaurin use. (2 cases were also databased in BNPV). Time to onset of reaction is 4 months and not available for another case. The dechallenge and rechallenge evidence is not available for these reports.
The European periodic assessment report from 2017 states that the phototoxic potential of this drug was evaluated in albino hairless mice with only mild skin reactions occurring after oral administration of midostaurin and UV-A or UV-A and UV-B irradiation.
A cumulative search global drug safety database of market authorisation holder (MAH) revealed 11 case reports of “photosensitivity or photo dermatosis conditions”. MAH assessed the causal relationship for these cases as “unlikely” based on presence of multiple co-suspected drugs, concomitant medications which are known to cause photosensitivity and had an unreasonable temporal relationship for the onset of reactions and presence of a prior history of photosensitivity.
There is no mention of photosensitivity in the product labels for midostaurin, but in a French compassionate use programme involving 28 patients, 25% of the patients reported an occurrence of photosensitivity reactions following treatment with midostaurin, strengthening the suspicion based on the reports found in BNPV and VigiBase.
A Drug Class Effect:
The other protein kinase inhibitors that have the potential for phototoxicity in their respective product labels are erlotinib, nilotinib, pazopanib, vandetanib, afatinib and vemurafenib in which the respective product labels are updated with this safety concern.
Advice to Healthcare professionals/consumers:
The measures of minimization this adverse reaction include extensive sun protection, extensive protective measures which included hat, long gloves, sunscreen of 50+ strength.
Patients should be informed about these hypersensitivity reactions so that they are able to take drugs of this profile without being exposed to unnecessary harm.