Metformin is a prescription drug and a first line medication used to control high blood sugar in patients with type 2 diabetes.
N-Nitrosodimethylamine is a probable human carcinogen which is not harmful when present within the permitted limits. Exposure to some level of nitrosamines is quite common, as these nitrosamines presence are common in water and foods, including cured and grilled meats, dairy products, and vegetables. As per FDA, the permissible daily intake limit for NDMA level is 0.096 micrograms or 96 nano grams. Exposure to NDMA above the acceptable levels or prolonged exposure (long term exposure) to NDMA in the body tissues might cause tumour formations. There had been significant major drug recalls happened due to the presence of higher levels of NDMA and the potential for formation of NDMA from the drug, based on its chemical structure.
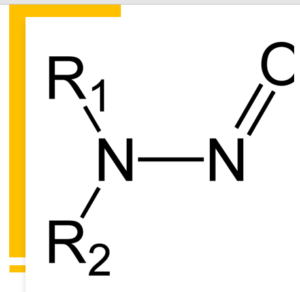
N-nitrosodimethylamine (NDMA) impurity
Investigation on presence of NDMA in Metformin
Since last year (2019), the U.S. Food and Drug Administration started investigating the presence of nitrosamines, N‑Nitrosodimethylamine (NDMA) for some types of drugs in collaboration with regulatory counterparts around the world, which has set internationally-recognized acceptable daily intake limits for nitrosamines. If drugs contain levels of nitrosamines above the acceptable daily intake limits, FDA recommends these drugs be recalled by the manufacturer as appropriate.
Angiotensin II receptor blockers (ARBs) (blood pressure and heart failure medicines), ranitidine, Nizatidine (heartburn medicines) were being recalled by pharmaceutical companies in many countries due to the identification of NDMA levels higher than the specified limit level.
On 05-Dec-2019, FDA released a statement on the presence of NDMA within the normal range in metformin extended release formulations outside the US.
On 03-Feb-2020, FDA posted the laboratory test results for NDMA presence, stating that “ NDMA levels in some metformin products approved in the U.S. FDA has determined that the levels of NDMA in metformin products tested range from not detectable to low levels.” However, FDA continued the investigation/testing for NDMA levels in metformin and other potential medications.
On 28-May-2020, FDA released an alert for health care professionals and patients on the presence of nitrosamine impurities (higher than the permissible/acceptable levels) in certain metformin extended-release (ER) products.

FDA has recommended the respective manufacturers of these extended release formulations of metformin to initiate a voluntary recall. FDA asked all manufacturers of metformin containing ER products to evaluate the risk of presence of excessive NDMA in their ER products and to test each batch before it is released into the U.S. market.
Reason for NDMA formation:
There are multiple reasons for the presence of NDMA in drugs. The possible sources found which could be related to NDMA formation are the listed below:
- Drug’s manufacturing process
- Molecular/Chemical structure of drug
- Storage and Packing conditions
- Nitrosamines, including NDMA, can be formed inside the body during the processing of food (digestion) or metabolism of drugs.
The US-FDA is collaborating with international drug regulatory agencies to investigate the possible sources of NDMA formation. A systematic approach is being followed by FDA for the identification of medicines having NDMA with higher than the permissible levels and initiating recall procedures for those drugs from the market.
Key Information for Patients:
- NDMA levels are not identified in Metformin immediate release (IR) formulations which are most commonly prescribed type of metformin medication compared to ER formulations.
- If patients with type 2 diabetes stop taking the metformin prescribed by their physician, it could cause potential life-threatening risk to their health or Potential hospitalizations for hyperglycaemia and ketoacidosis. So, Patients should continue taking metformin tablets even after recalls occur, until they consult with their health care professional who can prescribe an alternative medication.
Key Information for health care professionals:
- NDMA levels are not identified in Metformin immediate release (IR) formulations which are most commonly prescribed type of metformin medication compared to ER formulations. Also, NDMA is not identified in the samples of metformin active pharmaceutical ingredient
- The FDA recommended health care professionals to continue prescribing metformin to diabetic patients when clinically appropriate.