The following topics are covered as fundamental concepts in ICSR.
Key Definitions
Adverse Drug Event (AE): Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. An AE can therefore be any unfavourable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal (investigational) product, whether or not considered as related to the medicinal or investigational product.
Adverse Drug Reaction (ADR): An unwanted or harmful reaction experienced following the administration of a drug or combination of drugs under normal conditions of use and is suspected to be related to the drug. An ADR will usually require the drug to be suspended or discontinued or the dose reduction.
Day “0”/Initial Receipt date/Regulatory clock start date: The date of initial awareness/receipt date of a valid ICSR by a company/company’s representative, (Irrespective of weekend/holiday) is considered as “Day 0”. It is the start date of regulatory timeline for a case.
Company received date/Safety Received date/Central received date: It is the date when the Pharmacovigilance (Drug safety) department has received the valid ICSR information.
Country of Incidence: The country where the AE/ADR experienced by a patient irrespective of the country of reporter.
Regulatory timeline: The stipulated time period based on the type of ICSR defined by respective regulatory authority for submission of a case from its Day 0 to regulatory body.
ICSR validity criteria
The following four elements are considered for assessing the validity of an ICSR
- Identifiable patient,
- Identifiable reporter
- A suspect drug and
- An adverse event or adverse drug reaction.
Identifiable reporter : Any identifiable information for reporter such as qualification (e.g. physician, pharmacist, other healthcare professional, lawyer, consumer or other non-healthcare professional), name, initials, or address (e.g. reporter’s organisation, department, street, city, state or province, postcode, country, email, phone number).
Identifiable patient: Any identifiable information for patient such as initials, date of birth, age, age group, gestation period, or gender, medical record number (from general practitioner, specialist, hospital, or investigation), or any specific patient identification number.
A suspect drug: The generic name or brand name of the at least a single suspect drug/medicinal product.
An adverse event or adverse drug reaction: The information pertaining to adverse event, adverse drug reaction with a suspicious causal association with medicinal product.
Incomplete/Invalid case: Any case with the lack of any of the above specified four elements does not qualify for as a valid ICSR.
An Invalid/Incomplete ICSR can become valid/complete, upon receipt of any of the missing information from reporter.
The activities of Pharmacovigilance commence from pre-marketing stage of developmental drugs to the post-marketing phase of approved and marketed drugs. The sources of obtaining the patient’s safety information of a medicinal product can be classified into two categories based on marketing status of medicinal product.
- Pre-Marketing sources: The safety information from clinical trial studies sponsored, conducted and supported by pharmaceutical manufacturer or sponsor. It is a regulatory obligation for sponsors to monitor the safety profile of development molecule and report the significant safety concerns (adverse drug reactions which are unexpected as per the reference safety information) to respective health authorities and local regulatory bodies within stipulated timelines by respective authority.
There are few subcategories which can come under pre-marketing source:
- International clinical studies: The clinical trail period covering from Phase I to Phase III, where the patients/healthy volunteers are under strict clinical observance for monitoring of adverse experiences of medicinal product under development.
- Compassionate use programmes: A special approval for a single patient/group of patients use having a disease with no satisfactory authorised therapies and who cannot enter clinical trials for the use of unauthorized or unapproved medicinal product which is not yet released into market.
2. Post-Marketing sources: After availing the marketing authorization for a medicinal product, Marketing Authorization Holder (MAH) is obliged to monitor the safety behavior of medicinal product for a significant period of time in a wide range of population exposure which included populations not exposed to medicinal product under interventional monitoring or clinical study, to make sure the proposed benefit risk profile of the medicinal product to the regulatory authority is consistent with no significant variations. The following are potential sources of safety information for a medicinal product after marketing.
- Literature: The case study reports which originate from a health care professional or research professional in hospitals, research centers, post marketing studies and significant review articles (systematic and meta-analysis) published in either well established global medical databases (Medline, Embase) or from local un-indexed literature journals.
- Legal: The safety information reported by lawyer on behalf of individual patient or group of patients concerned with occurrence of adverse reactions associated with medicinal product use.
- Post marketing studies, registries and programmes: As part of routine and additional pharmacovigilance activities and risk minimization measures, MAH are obliged to conduct the post marketing studies, registries and programmes to address specific safety concern identified from public exposure and to present the positive benefit risk ratio of their respective medicinal product to regulatory authority.
- Spontaneous reports: It is a broad range of source which provides safety information either from health care professional (HCP) or directly from consumer. It could be in two ways as follows:
- Voluntary/Passive reporting: The safety information could be reported directly to health authorities (HA) from HCP and consumer by HA specific adverse event form or through toll free number provide in HA website. The HA would share the same information to respective drug manufacturer for necessary action required or for processing in their safety database.
- Mandatory/Active reporting: The safety information is reported to manufacturer’s drug safety center by HCP or consumer either by submitting the company specific adverse event/reaction form for spontaneous reporting or by online reporting platforms in MAH’s website or by reporting through toll free number dedicated for drug safety. MAH submits the same safety information to respective HA within the stipulated timelines.
Based on the sources and the type of data collection, ICSRs are classified into two categories.
- Solicited ICSRs: The ICSRs which where originating from a organised data collection systems are classified as solicited type. The following types of ICSRs comes under solicited category.
- Interventional Clinical Trail/Studies
- Non-Interventional Clinical Trial/Study
- Non-Interventional Programmes
- Patient Registries
- Patient support & Market research programmes
- Investigator Initiated clinical Trails, Compassionate use programmes etc.,
2. Non-solicited ICSRs: The ICSRs, which are originating from an Un-organised data collection systems are classified as Non-solicited type. The following types of ICSRs comes under Non-solicited category.
- Literature Abstracts/Articles
- Legal Cases reported by Lawyer on behalf of consumer (client)
- Health authority case reports
- Spontaneous reports from health care professionals and directly from consumers
- Social media reports-Any ICSR information posted on a social websites like Facebook, Twitter etc.,
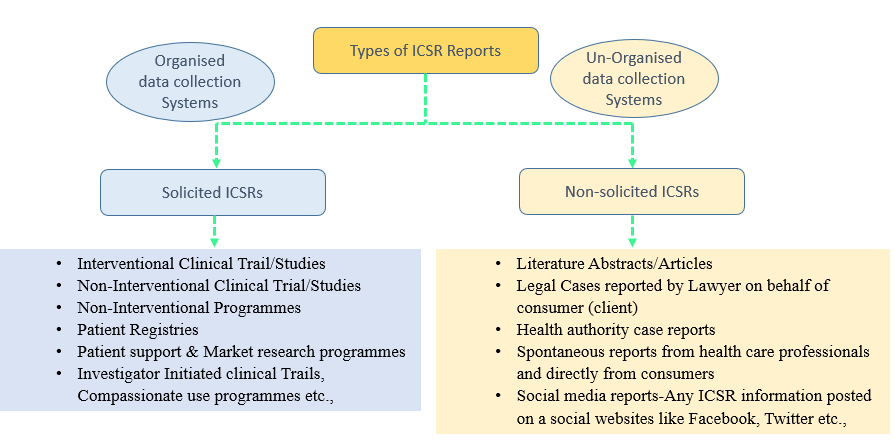
Overview on Types of ICSRs based on their source