Triage/Intake of ICSR information
Listedness/expectedness assessment
Action Items/Request for Follow up information
Some more important information into safety database
Lets look into each concept in detail
Intake/Triage of ICSR information
The ICSR information is first received by Intake team followed by triaging the case.
Below activities would be performed during this phase.
- Receiving the source document(s) (containing safety information) and sending acknowledgment to the sender for the same
- Assessing/Determining Validity of ICSR case
- Querying/Follow up with sender for the missing information.
- Entry of basic elements of ICSR (like reporter’s country, any one of patient demographics, suspect drug, and AE/ADR information) into the safety database.
- Prioritizing the case based on seriousness determination/causality and notifying the relevant stakeholders for expedite action.
- Splitting of cases (In case of multiple, summary of cases from a literature source) into separate ICSR(s).
Criteria: The below conditions are considered six different level of seriousness criteria.
Fatal/Death:
The outcome of an adverse drug event is death. i.e, patient died due to adverse drug event or reaction.
Life-threatening:
The patient is at substantial risk of dying at the time of the adverse drug event (or) Use of (or) continued use of the drug might have resulted in the death of the patient.
Requiring hospitalization (Inpatient and prolongation of existing hospitalization):
Patient admitted to hospital due to adverse drug event or patient’s hospitalization prolonged due to adverse drug event.
Results in persistent or significant disability or incapacity:
A substantial disruption of a person’s ability to conduct normal life functions due to adverse drug event. If any adverse event resulted in a significant, persistent, or permanent change, impairment, damage or disruption in the patient’s body function/structure, physical activities and/or quality of life.
Results in a congenital anomaly (birth defect):
Adverse drug events identified in newly born children (Any skin reactions, defects in the body of baby etc.,) suspecting to be caused by drug exposure during pregnancy or before conception.
Medically significant (or) Other Important medical events
Considered serious, because some medical treatment/surgical intervention would be required to prevent one of the above 5 serious conditions.
Based on seriousness, an AE/ADR can classify into two categories.
- Serious AE/ADR: Any AE/ADR which is having any one of above listed seriousness criteria (or sometimes combinations of serious criteria) is considered as serious AE or serious ADR.
- Non-Serious AE/ADR: Any AE/ADR which is “Not” associated with any one of the above listed seriousness criteria is considered as a non-serious AE or non-serious ADR.
- The assessment of relationship between a drug treatment and the occurrence of an adverse event.
- The determination of whether there is a reasonable possibility that the medicinal product is causally related to the adverse event.
- It is the key factor for identification of new signals, measuring the strength of evidence, and in evaluating benefit risk profile of drugs.
The assessment of causality is a common routine procedure in pharmacovigilance, which is done at different levels which included physicians, investigators, professionals working in drug safety department of a pharmaceutical company and national health authorities which can assist in taking regulatory decisions.
Methods of causality assessment:
There are several methods published to perform causality assessment. However, there were no internationally agreed upon standards or criteria for assessing causality in individual case safety reports.
Below two methods are widely used for assessing causality globally
- WHO UMC causality assessment
The World Health Organisation (WHO) and Upsala Monitoring center (UMC) at Sweden has developed a system for causality assessment in consultation with the National Centers participating in the International Drug Monitoring Programme.
It is meant as a practical tool for the assessment of causal relationship in ICSRs.
It is basically a combined assessment considering the clinical-pharmacological aspects of the case history and the quality of the documentation of the observation.
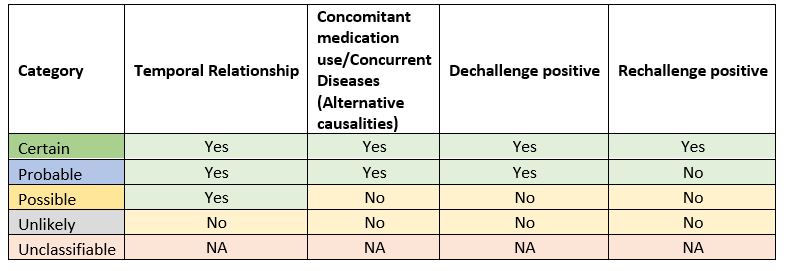
The following causality terms are defined based on their respective assessment criteria.
| Causality term | Assessment criteria |
| Certain | Event or laboratory test abnormality, with plausible time relationship to drug intake Cannot be explained by disease or other drugs. Response to withdrawal plausible (pharmacologically, pathologically). Event definitive pharmacologically or phenomenologically (i.e. an objective and specific medical disorder or a recognized pharmacological phenomenon) Re-challenge satisfactory, if necessary. |
| Probable / Likely | Event or laboratory test abnormality, with reasonable time relationship to drug intake. Unlikely to be attributed to disease or other drugs. Response to withdrawal clinically reasonable Re challenge not required. |
| Possible | Event or laboratory test abnormality, with reasonable time relationship to drug intake. Could also be explained by disease or other concomitant drugs. Information on drug withdrawal may be lacking or unclear. |
| Unlikely | Event or laboratory test abnormality, with a time to drug intake that makes a relationship improbable (but not impossible). Disease or other drugs provide plausible explanations. |
| Conditional / Unclassified | Event or laboratory test abnormality More data for proper assessment needed, or Additional data under examination. |
| Unassessable / Unclassifiable | Report suggesting an adverse reaction. Cannot be judged because information is insufficient or contradictory Data cannot be supplemented or verified. |
Assessment criteria: Below key points will aid in ease understanding of various criteria for assessing causality.
- Temporal relationship: It is the time relationship between the drug administration (Date of therapy start date, therapy duration) and occurrence of adverse event (Event onset date/date of initial symptoms observed).
- Abnormal Laboratory tests: There are some adverse event incidences which cannot be ruled out from the causal relation with drug based on unusual or abnormal values in the laboratory investigation.
- De-challenge: The assessment of the outcome of adverse event followed by suspension/withdrawal of drug/reduced dose of drug in response to the adverse event. Based on outcome of AE, it is further classified into two categories:
- Positive Dechallenge: The complete or partial resolution of adverse event followed by suspension/withdrawal of drug/reduced dose of drug
- Negative Dechallenge: There is no change in the outcome of AE. It is persisting irrespective of drug suspension/withdrawal and reduced dose.
- Re challenge: This is applicable in the positive Dechallenge scenario. The re‑introduction/restarting of drug therapy after the event resolution. It also can be classified into below two categories:
- Positive Re challenge: Re-occurrence of adverse event after restarting of drug.
- Negative Re challenge: Adverse event has not recurred even after drug restart.
- Alternative causality: Other contributory factors for the cause of adverse event. Below are the possible contributors attributed to an AE.
- Medical conditions: Underlying concurrent medical conditions of patient (e.g., diabetes, heart diseases, autoimmune disease etc.,) or past medical history and prior or ongoing surgical procedures.
- Other medicinal product use: Concomitant medication details and past drug history.
- Social life: Alcohol use, smoking (both history & concurrent use), obesity, diet, profession etc.,
- Risk factors: Age (both paediatric and geriatric patients), hepatic and renal impairment patients etc.
Below table will aid in identification of causal criteria based on the assessment of various factors.
2. Naranjo causality assessment
In the year 1991, Naranjo and co-workers from the University of Toronto developed the Adverse Drug Reaction (ADR) Probability Scale to determine the likelihood of whether an ADR is due to the medicinal product rather than the result of other contributory factors.
It is often referred to as the Naranjo Scale which is simple to apply and widely used.
ADR probability scale: The Naranjo Algorithm, or Adverse Drug Reaction Probability Scale, is used to assess whether there is a causal relationship between an adverse drug experience and a drug using a simple questionnaire to assign probability scores.
The questionnaire scale consists of 10 questions that are answered as either “Yes”, “No”, or “Do not know”. Different point values (-1, 0, +1 or +2) are assigned to each answer.
Listed below are the 10 questions:
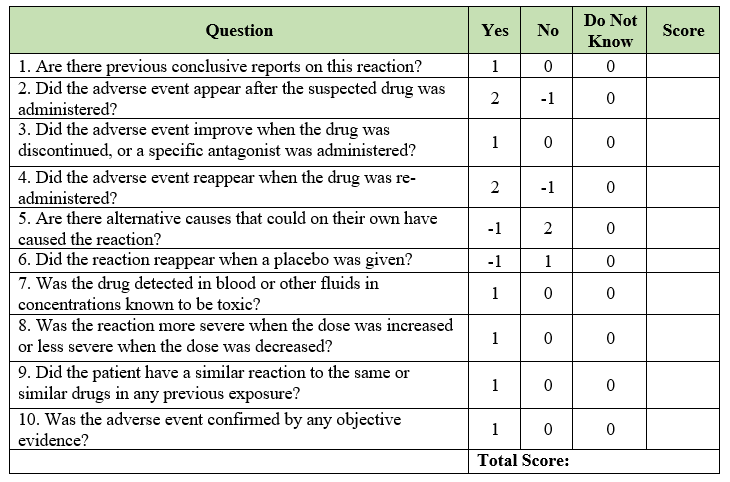
The total scores range from “-4 to +13”
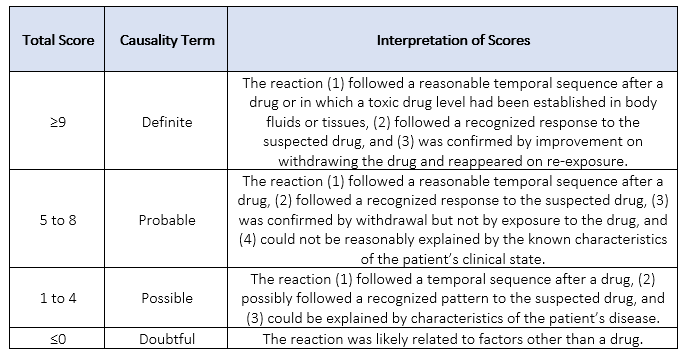
Based on the total score, causality is assessed, and the strength of relationship is defined.
MedDRA (Medical Dictionary for Regulatory Activities) is a standardized medical terminology tool which is rich and highly specific and facilitates sharing of regulatory safety information internationally for medical products used by humans.
It was developed by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) in the late 1990.
Scope of products covered by MedDRA: Pharmaceutical (medicinal products) products, biological prodcuts, vaccines, and drug-device combination products. MedDRA is used worldwide by national regulatory authorities, pharmaceutical companies, clinical research organisations and health care professionals which allows better global protection of patient health.
Updates to MedDRA: Released twice in a year by MSSO (Maintenance and Support Services Organization). First release in the month of March which includes completed updates and second release in the September month simple revisions.
In ICSR case processing, MedDRA dictionary is used for coding medical conditions, adverse event terminologies into the safety database.
Drug safety databases (like Argus and Arisg) contain integrated MedDRA dictionaries for facilitating case processors to capture the reported adverse event(s) or medical condition(s) from the source document(s).
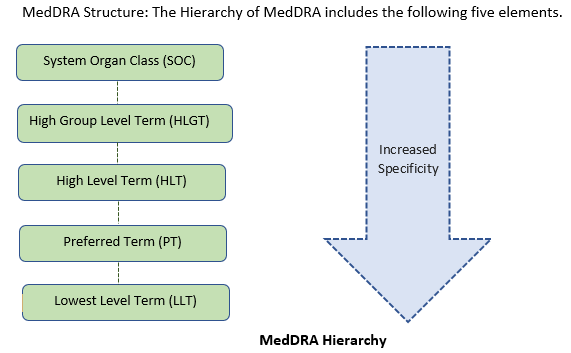
Lowest level term (LLT):
It represents the clinical observations, reported verbatims of adverse experiences, special conditions by reporter. LLTs are highly specific verbatims and each LLT is linked to only one PT (Preferred term) and users can retrieve data at the most specific level of the terminology.
LLTs facilitate entry of reported verbatims and promote consistency by decreasing subjective choices made at this stage. LLTs may also be used as the basis for auto-encoding. The verbatim terms used under LLT can be vague, ambiguous, truncated, abbreviated, out-dated, or misspelled carry a non-current flag.
Preferred Terms (PT):
Preferred term is a single medical concept (distinct descriptor) for signs, symptom, clinical diagnosis, diseases, therapeutic indication, investigation, surgical, or medical procedure, and medical, social, or family history characteristic.
PTs are not ambiguous and as specific and self-descriptive as possible in the context of international requirements. A PT must have at least one LLT linked to it. One PT can encompass many lowest level terms and there is no limit to the number of LLTs that can be linked to a PT. When a new PT is added to the terminology, an identical LLT is created automatically for case processing purposes. PTs are subordinate to Highest level terms (HLTs).
A PT must be linked to at least one SOC. A PT can be linked to as many SOCs as is appropriate. It can only be linked to each SOC via one HLT HLGT SOC route.
Each PT has a primary SOC that determines under which SOC the term appears in cumulative data outputs.
High Level Term (HLT):
High level terms are intended for data retrieval and presentation purposes and they are a grouping level and are not intended to be a coding level.
An HLT is a super ordinate descriptor for the PTs linked to it. It is an inclusive category which links PTs related to it by anatomy, pathology, physiology, etiology, or function.
HLTs are subordinate to HLGTs. An HLT must be linked to at least one SOC via an HLGT. It can only be linked to a single SOC via one route (i.e., linked to only one HLGT per SOC). All HLTs linked to a HLGT will appear in every SOC to which the HLGT is linked.
High Level Group Term (HLGT)
High Level Group Term is a superordinate descriptor for one or more HLTs related by anatomy, pathology, physiology, etiology, or function. Again, HLGTs are also intended for data retrieval and presentation purposes like HLT.
Both HLT and HLGT are used to aid retrieval by broader concepts. HLGTs are subordinate to SOCs. An HLGT must be linked to at least one SOC and to at least one HLT and there is no limit to the number of SOCs to which an HLGT can be linked.
System Organ class (SOC):
The highest level of the hierarchy that provides the broadest concept for data retrieval. It comprises groupings like below:
• Etiology (e.g., SOC Infections and infestations)
• Manifestation site (e.g., SOC Gastrointestinal disorders)
• Purpose (e.g., SOC Surgical and medical procedures)
A SOC is related directly (superordinated) to at least one HLGT with no restriction on the number of links to HLGTs.
Example of MedDRA coding:
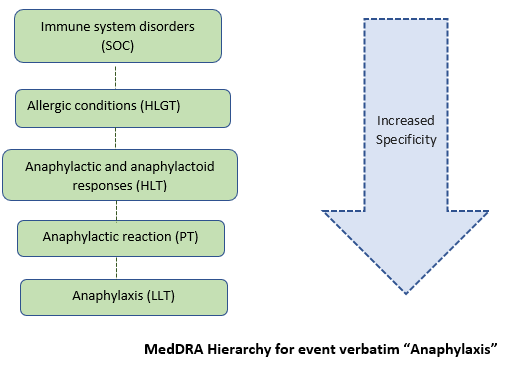
In an ICSR, Anaphylaxis is the event verbatim reported for a patient. When the case processor enters this event “Anaphylaxis” into the MedDRA dictionary search, It will automatically pull into “Anaphylactic reaction” under PT (broader term than Anaphylaxis) followed by grouping under HLT and HLGT as “Anaphylactic and anaphylactoid responses” and “Allergic conditions” respectively under the single umbrella-SOC “Immune system disorders”.
Below picture depicts example for better understanding.
Medicinal products or Drugs are coded using drug dictionaries integrated in the drug safety database
The Drug Safety databases comprises of two drug dictionaries.
- WHODD- For Coding of Drugs which are not manufactured by the Sponsor
- CPD-For Coding of Drugs which are manufactured by sponsor
- WHO DD (Word Health Organisation Drug Dictionary)
WHO DD is the dictionary used for coding medicinal products worldwide by pharmaceutical companies into their drug safety databases used in the routine pharmacovigilance practices. WHO DD is mandated by many national regulatory authorities to use by pharmaceutical companies.
WHO DD is the international reference for medicinal product information maintained by Uppsala Monitoring Centre (UMC) at Sweden.
The dictionary is used to identify drug names and evaluate medicinal product information, including active ingredients and product’s anatomical and therapeutic classifications, from nearly 150 countries.
In an ICSR, the medication details reported for patient from the source document which included Suspected drugs, concomitant medications and past drug medications (Which are not manufactured by company) used by the patient, all are entered into the product information tab of the safety database using the WHODD.
WHO DD has 3 parts-
Drug Record Number (DrugRecNo): It consists of 6 characters which uniquely identifies active moieties, regardless of salt form or plant part and extract.
Sequence number (Seq1): It is used to uniquely identify different variations (e.g. salts and esters), plant parts and extraction methods, thereby defining active substances or a combination of active substances
Sequence number (Seq2): It uniquely identifies the name of the record in WHO Drug dictionary.
Anatomical Therapeutic Chemical (ATC) Classifications system:
WHO Drug records are classified with at least one code from Anatomical Therapeutic Chemical Classification System.
It is an internationally accepted classification system for medicines that is maintained by the World Health Organisation (WHO).
WHO assigns a unique code for each active pharmaceutical ingredient contained in the medicinal products based on their therapeutic indication. ATC code comprises of 7 elements which are combinations of letters and numbers.
Using the ATC code, active substances are classified in groups at five different levels according to the organ or system based on their mechanism of action, therapeutic, pharmacological, and chemical properties.
Let us look into an example for more understanding.
ATC code for Pantoprazole: This molecule is present in three different medicinal products. Each medicine has its unique ATC code assigned by WHO as below.
- A02BC02 pantoprazole
- A02BD04 pantoprazole, amoxicillin and clarithromycin
- A02BD11 pantoprazole, amoxicillin, clarithromycin and metronidazole
Now, let us have a look on A02BC02 pantoprazole detailed anatomical classification.
|
Level |
Classification |
ATC Code |
|
Main Group |
Alimentary tract and metabolism |
A |
|
Therapeutic Group |
Drugs for acid-related disorders |
A02 |
|
Pharmacological subgroup |
Drugs for peptic ulcer and gastro-oesophageal reflux disease |
A02B |
|
Chemical subgroup |
Proton pump inhibitors |
A02BC |
|
Active substance |
Pantoprazole |
A02BC02 |
ATC code is useful in identification of drug’s defined daily dose which is used in drug exposure calculations during preparation of PBRER/PSUR.
- Company Product Dictionary (CPD):
During Case processing, the Suspected drugs, concomitant medications, and past drug medications which are manufactured by the company should be coded using the company product Dictionary.
After coding, it will auto populate the additional details like ANDA (abbreviated new application number) for generic medicines or NDA (New Drug Application number) for Brand medicines or IND (Investigational New Drug application number) for investigational drugs which is specific for the respective manufacturer of the medicinal product.
Listedness/Expectedness assessment
This concept involves determining whether any adverse drug reaction is already known for the medicinal product.
The term “Listedness” is used for during assessment for the “Marketed products”.
The term “Expectedness” is used during assessment for “Developmental drugs or Investigational molecules”.
Before going into definition of what exactly listedness and expectedness mean, we will know about the “Reference Safety Information”.
Reference Safety Information (RSI)
For Developmental Drugs/Investigational molecules:
Investigational Brochure (IB): During development phase of pre-marketed medicines which are not “Yet” approved, the information pertaining to Safety Experience of molecule under investigation gained during the study period or clinical phase development is documented in a detailed manner. The name of the document used for this purpose is “Investigational Brochure” (IB).
So, IB is the reference safety information (RSI) document for assessing whether any adverse reaction is already known or not.
The term used here is Expectedness. If any adverse reaction is already encountered by any patient for that molecule under investigation and it is “Well Documented” in the IB, then adverse reaction is considered as “Expected”. If it is a strange adverse drug reaction and “Not Well Documented” in IB, then it is classified as “Unexpected”.
For Marketed Medicinal products:
After attaining authorization and approval for marketing a medicinal product, Sponsors will prepare a Package Information Leaflet (PIL), Prescribing Information document.
Each Marketing Authorisation Holder (MAH) maintains a reference safety document specific to the region. Also, each national regulatory authority maintains product specific monographs which included drug safety information.
Some of the labelling documents presented below-
Summary of Product Characteristics (SmPC): The RSI for European Medicines Agency (EMA) region or any European country specific document.
United States Prescribing Information (USPI): Country Specific Document for United States of America.
Japanese Prescribing Information (JPI): Country Specific Document for Japan.
Canadian Monographs (CM): Country Specific Document for Canada.
The terms Listed/Unlisted or Labelled/Unlabelled used here for marketed medicinal products. If any adverse reaction experienced by patient is Well Documented in its corresponding Reference Safety Information (RSI) for that medicinal product, then adverse reaction is considered as “Labelled”. If it is a strange adverse drug reaction and “Not Well Documented” in its respective RSI document, then it is classified as “Unlabelled”.
A company’s common core data sheet (CDS) or Company’s Core Sheet Information (CCSI) are the “Global Reference Safety Information” documents maintained by the sponsor for the complete safety information of medicinal product gained from patient experience globally and updating the same whenever required.
If any adverse reaction is well described in the CDS/CCSI, it is classified as “Listed” else it is “Unlisted”.
So, based on the type of medicinal product (developmental or marketed) and type of RSI (IB, CCDS, CCSI, USPI, SmPC etc.,) different verbatims are used (Expected/Listed/Labelled) during assessment.
Here, I am speaking of Well Documented and Not Well Documented in the above classifications. Now let us see what exactly the Well Documented means.
Definition: Any AE/SAE or ADR/SADR is considered Well Documented only when its “Nature, Severity, Specificity and Outcome” are Consistent with available safety information presented in the Reference Safety Information (RSI) document.
In Detail-
Nature: If an adverse event or reaction term or verbatim nature is well described in the RSI document. For example, “Keratoacanthoma” is the event verbatim reported for a patient from source document. The information pertaining to this reported event is well described in relevant section (Adverse reactions section) RSI. If RSI contains only information pertaining to skin cancer, then event Keratoacanthoma considered as not well documented in the RSI.
Severity: When the severity of reported adverse event from the ICSR case is not consistent with presented severity for the same event in the RSI document. For example, an event “Headache” described in the RSI section with the severity of Mild or Moderate. Now, we have received an adverse event of “severe headache” from the ICSR source document. Then this severe headache is considered as Unexpected/Unlisted/Unlabelled (as per type of RSI).
Specificity: Based on the specificity of adverse reaction reported for a patient in an ICSR, it can be considered “Not well documented”. Example: we have an ICSR with the event “Right leg oedema”. But in the RSI, left leg oedema is described. Then our event Right leg oedema could be classified as Unexpected/Unlisted/Unlabelled (as per type of RSI).
Outcome: An adverse event outcome plays a key role in listedness assessment. For example, In our ICSR case, patient died due to pneumonia (i.e., pneumonia with fatal outcome). Whereas in the RSI, only “pneumonia” is described. In this scenario, “fatal pneumonia” could be assessed as Unexpected/Unlisted/Unlabelled (as per type of RSI).
Sometimes, based on change in frequency of expected/listed adverse event occurrences could be classified as unexpected/unlisted. (We will discuss more on this in Aggregate reporting and signal evaluation concepts).
Expectedness/Listedness assessment is crucial in the pharmacovigilance which could determine expeditious nature of an ICSR. (we will discuss this more under regulatory timelines concept).
This is the most critical and important step in ICSR-case processing. Because, it provides the picture of entire ICSR which include the patient’s safety experience pertaining to company’s medicinal product.
The main objective of narrative writing is summarisation of relevant patient safety information in a chronological manner which projects as the story of patient’s experiences pertaining to company’s medicinal product exposure.
The process of writing a narrative is initiated when the all the information is coded/entered into the safety database.
Most of the safety databases (Arisg, Argus etc.,) contain auto-narrative feature which generates a automated narrative based on the safety information entered into the database by the processor.
Most of the safety databases contain auto-narrative feature which generates a automated narrative based on the safety information entered into the database by the processor.
After generating auto-narrative, its case processor responsibility to optimize the narrative in a company specific format in a chronological order of patient experience and necessary amendments before routing the ICSR to next level for review.
Here are some of important tips while drafting a patient safety narrative.
- Use Sponsor specific style
- Should be written in the past tense
- No redundant lines or information
- Expand the abbreviations
- Checking for grammar, spellings, and format
- Should be concise and precise
- Maintaining chronological order for patient experience.
Below, we have provided a generalized format/structure for narrative for better understanding.
Structure of Narrative
It can be divided into five different parts-
- Introductory paragraph
- Second Paragraph
- Main Paragraph
- Causality statements
- Conclusion
Introductory paragraph: An ICSR narrative starts with introductory paragraph which comprises of case ID (Unique ID given to an ICSR), type of ICSR (based on source), Initial received date (Regulatory Day 0), type of reporter (Health care professional, Patient etc.,), patient demographics (patient’s age, gender, ethnicity, country etc.,) followed by the title of clinical study (Applies to only CT (clinical trial) ICSR cases.
Example:
Case number XXXXX is an initial literature case report received on 05 May 2020 from a health care professional pertaining to a 38- year-old male patient from the United States who experienced liver injury while on paracetamol.
Second Paragraph.
Second paragraph of narrative comprises the following elements
- Patient’s medical history and drugs – Past medical conditions experienced by the patient and resolved at the time of company’s product administration/adverse event onset. Previous medicine history used by patient and which are stopped at the time of suspected drug administration.
- Patient’s Concomitant medication and Concurrent conditions – The current medical conditions present in the patient at the time of suspected drug administration and the current medication detail used by patient concomitantly with suspected drug.
- Apart from medical conditions and drugs information, social history (like alcohol use, smoking etc.,) of the patient also included here which could support in causality assessment.
Example:
The patient’s current conditions included diabetes mellitus, severe headache, cervical spine degeneration, hypertension, gastritis. The patient’s concomitant medications included metformin for diabetes, diclofenac for swelling and headache, iohexol for cervical spine degeneration, metoprolol for hypertension and pantoprazole for gastritis. The patient’s medical history included anaemia and pneumonia. The patient’s past medicine history included folic acid supplements and Azithromycin. The patient’s history of alcohol use and smoking were not reported by the reporter.
Main Paragraph:
This is the core paragraph which comprises of highly essential elements like below.
- Therapy details- Suspected drug therapy details which included, name of suspected drug date of administration, dose, strength, route of drug administration and indication for use.
- Event details- Name of the event, onset date of event, treatment given for the event, seriousness of event, sometimes it is required to mention the signs and symptoms experienced by the patient before the final diagnosis in a chronological order with the integration of relevant laboratory investigation details, and treatment medicament details.
- Action taken with suspected drug due to the event – Incorporating the action taken for the suspected drug (which could include drug suspension, dose reduction, or complete withdrawal) in response to or due to the adverse drug event.
- Outcome of Adverse event – The outcome for the event at the time of receipt of the report, which could be resolved, resolving, not resolved, or not reported.
Example:
On 18-Apr-2020, The patient-initiated therapy with paracetamol of unknown dose via oral route for severe fever. Since 20-Apr-2020, Patient experienced Pain in your upper right abdomen, nausea, vomiting and abdominal swelling. On 23-Apr-2020, Patient admitted to hospital for treatment. On 24-Apr-2020, liver function tests were performed, and the patient was diagnosed with liver Injury. On the same day, paracetamol use was stopped due to the event, liver Injury. Physician reported that patient was recovering from the event liver injury.
Causality statements:
It comprises of both the reporter causality and company causality assessments.
Reporter causality: The causal relationship reported by the reported (either related, unrelated, not sure, or not reported) is mentioned.
Company causality: The assessment of causality by the company (Drug safety expert) is mentioned.
Example:
The physician assessed the causal relationship between paracetamol and liver injury as ‘related’.
The company assessed the causality as ‘related’ for the event liver injury with paracetamol use.
Conclusion:
It includes that additional information might require to this ICSR for complete assessment. OR
No further information available.
When a follow up is received for the same ICSR, the additional safety information would be entered into the safety database and it would be amended accordingly in the narrative.
A statement is added at the end of narrative specifying the additional safety information received, added, and appended in the narrative.
For ICSRs from Literature source: Author’s comment and citation details would be added at the end of narrative.
Action Items/Request for Follow up information
It includes the following items:
- Raising queries for discrepant information
- Requesting for additional information
- Requesting for clarity in source documents with indecipherable writings
When we receive an ICSR with complete information and with no discrepancies within the reported information from a clear source document. Then there would be no requirement of the above action items.
Ideally it is very rare to receive ICSR with the complete and non-discrepant information. So, in general we receive ICSRs from different sources which requires additional information and clarity for complete assessment.
Below, we have highlighted the possibilities of raising general queries for an ICSR.
For Incomplete ICSRs: Requesting for missing information to qualify the case as valid for further processing.
For Important information: Requesting for suspect drug therapy details (onset, dose etc,) event onset date, clarification of event verbatim, event outcome, action taken for suspected drug with respect to event, relevant laboratory details and treatment medications for the event.
For Additional information: Requesting for patient’s medical history, concomitant medications, concurrent conditions which are not reported in the ICSRs source document.
For Discrepant Information: Queries can also raise to resolve the discrepant information reported in the source document which might include discrepancy between event onset date, outcome, drug start date, action taken etc.,
Example: In an ICSR source document, under the event description section it was reported that event is ‘resolved’, whereas in the event details section it was marked as ‘not resolved’. Hence a query a need to be raised to resolve this discrepancy.
It is the case processor’s responsibility to identify the need/requirement for raising the query or following up with the reporter for additional and discrepant information.
The follow up should be initiated in a timely manner considering the regulatory timelines of a an ICSR. And the reminders for the follow ups (already sent to reported) is based on standard timelines specific for sponsor.
For Some special events and scenarios, it might be required for case processor to initiate follow up containing Targeted Questionnaire (TQ) for obtaining the more relevant and important information pertaining to the special safety topic.
Some more important information into safety database
Let us discuss some more important information for while ICSR case processing in relation to a safety database.
- General tab: The report type, classification, the initial received date (Day 0), reporter information (Name of reporter, contact details etc.,), medically confirmed status etc., would be entered into this tab of a safety database.
- Patient tab: The patient’s demographic information like age, initials, gender, date of birth, Height, weight etc., needs to enter the patient tab.
Self-Quality check (QC):
ICSRs are the building blocks for the Pharmacovigilance, which are used in analysis during the preparation of aggregate reports and performing signal evaluations.
ICSRs of less quality could lead to missing of identifying potential signals.
ICSRs of inadequate quality would create bad regulatory impression.
It is advisable to perform a self-review for checking the quality of a processed ICSR.
- Reviewing the “entered information” in different tabs of safety database by crosschecking the “reported information” from source document.
- Narratives- Safety Narratives are first line impression for an ICSR. Narrative should be written in concise and in medically cohesive manner.
Validation:
Before submitting the ICSR case to next level, it is always advisable to perform validation check to make sure the case is having all the regulatory fields processed with sufficient information.