Amneal Pharmaceuticals announced recall of nizatidine oral solution, 15 mg/mL, due to presence of potential levels of N-Nitrosodimethylamine (NDMA) impurity, (a probable human carcinogen).
Nizatidine is a similar drug to Ranitidine which is commonly used in the treatment of peptic ulcer disease and gastroesophageal reflux disease. It acts as antagonists for histamine (H2) receptor, thereby inhibiting stomach acid production.
Recalls for Ranitidine and Nizatidine products:
These drugs being recalled from national regulatory bodies of most developed countries globally and voluntarily from the product manufacturers. Even though, these medicines are well established in the market since decades, a new concern for patient safety risen with the identification of an impurity, NMDA at higher levels than FDA permitted (As per FDA, the permissible daily intake limit for NDMA level is 96ng).
The presence of this higher levels of NDMA is first identified by Valisure. Valisure is an online pharmacy currently licensed in 38 states of United States also with an analytical laboratory which analyse and performs quality tests for the medicines dispensing by them. During their routine analysis before giving medicines to consumer, they have identified NMDA for ranitidine containing products.
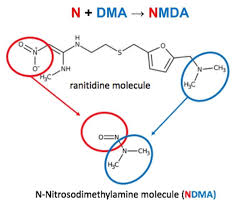
Valisure’s analysis revealed the ability of Ranitidine molecule to form NMDA at high efficiency. They initially conducted FDA recommended GC/MS (Gas Chromatography and Mass spectrometry) headspace analysis method, which involves heating in the GC/MS of 130°C oven incubation for 15 minutes which resulted in the highly efficient conversion of ranitidine to NDMA.
Valisure have conducted another experiment which is biologically relevant in 100mL reaction volumes with ranitidine tablet (150 mg). This experiment involved conditions for simulating the human stomach by using industry standard “Simulated Gastric Fluid” (50 mM potassium chloride, 85 mM hydrochloric acid adjusted to pH 1.2 with 1.25 g pepsin per liter) and “Simulated Intestinal Fluid” (50 mM potassium chloride, 50 mM potassium phosphate monobasic adjusted to pH 6.8 with hydrochloric acid and sodium hydroxide) and in combination with various concentrations of nitrite, which is commonly ingested in foods like processed meats and is elevated in the stomach by antacid drugs. The reaction time allowed to react is 1 hour. This experiment in simulated gastric fluids demonstrated significant NMDA formation from ranitidine molecule.
The proposed mechanism for Ranitidine degradation to NMDA:
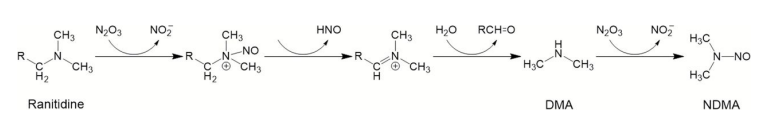
The other possibility of NMDA formation is enzymatic liberation of ranitidine’s DMA group from the molecule by dimethylarginine dimethylaminohydrolase (DDAH). This process can occur in other tissues as well apart from stomach. This liberated DMA could react either with nitrite group of molecule or with nitrite group freely circulating in the body and other pathways under weak acidic conditions present in kidney and bladder.
The carcinogenic nature of ranitidine in combination with nitrite was suspected in the year 1983, just two years after the drug entered the market, by the publication of in vivo study results in journal Carcinogenesis journal with the title “Genotoxic effects in rodents given high oral doses of ranitidine and sodium nitrite”.
A study by Professor William Mitch and his team at Stanford University in the year 2016 published alarming results which showed that healthy individuals (both male and female), had elevated amounts of NDMA in their urine (~40,000 nanograms) in the 24 hour period after ingestion of ranitidine 150 mg tablets, which is 4000 times more than the permissible level.
Nizatidine have similar molecular structure containing N, N dimethyl amine (DMA) group and nitroso group with the possibility of formation of NMDA. Valisure identified millions of nanograms and tens of thousands of nanograms of NDMA in ranitidine and nizatidine samples respectively.
As per the statement by Valisure and the published studies, NMDA production is not attributed to the manufacturing procedure of medicine, or storage conditions. It is directly attributed to the molecular structure itself.
The recall of these class of medicines is paramount for public safety. The channels for increasing the evidence pertaining to safety should be established for “established drugs” also in order to reduce the time of awareness from drug launch to the identification of potential safety hazards to public.
The time took for the ranitidine drug launch (1981) and the awareness to recall from market (2020) for its carcinogenic potential is 38 years!
In this period of 38 years, it is not known about the number of people globally affected with cancers (especially kidney and bladder cancers) attributed to the ranitidine use. If safety data collection methods for drugs are robust, many patients or general public can be protected from medicines with negative benefit to risk profiles.
No regulatory action has taken yet for these class of medicines by CDSCO. This might take some significant time to announce recall.