A serious type of recall for medical device class I has announced by FDA for the Boston Scientific Corporation IMAGER II 5F Angiographic Catheters. This class I device is used to provide a pathway to deliver contrast agents to blood vessels including carotid arteries during angiography.
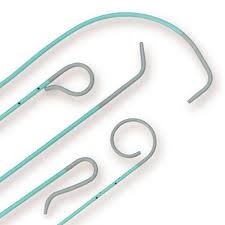
During the patient procedure preparation, potential for the catheter tip to become detached has observed which could lead to additional surgical procedure for the removal of catheter tip in the patient’s blood vessel and which in turn causes prolonged hospitalization. This device also has the potential for serious adverse events which include embolism (blood flow obstruction), stroke, or death.
FDA received nine reported injuries pertaining to this medical device. A total of 6,130 devices involved in this recall which were distributed between 16-July-2018 and 26-Nov-2019, with expiration dates that range from 8-Jun-2020 through 2-Mar-2021.

On 11-Feb-2020, Boston Scientific Corporation informed the respective customers of the affected lot numbers and instructed to remove any affected lots in the hospital inventory, to stop using any product with the affected lot number, to complete the verification form and include the quantity of units from each affected lot and to return the affected lots to Boston Scientific Corporation.
The customers having questions about the notification of this device recall can contact their local sales representative or [email protected] for additional information.
Reference: