On 11-Jun-2020, a nationwide recall of one lot of Metformin Hydrochloride ER Tablets USP, 500 mg [Lot Number: G901203] issued by Lupin Pharmaceuticals, Inc. due to the detection of N-Nitrosodimethylamine (NDMA).
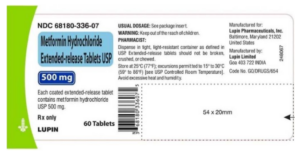
Recalled Product-Metformin ER Tablets USP (generic equivalent of Fortamet®), 500 mg, lot G901203
Metformin Hydrochloride is indicated as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes mellitus. NDMA is identified as a probable human carcinogen (a substance with potential to cause cancer) based on results from laboratory tests. With the release of alert of NDMA presence in Metformin extended release (ER) formulations, U.S. Food and Drug Administration recommended the respective manufacturers of the tested lots to initiate voluntary recall. Lupin is fourth pharmaceutical firm for issuing this recall procedure in continuation to 3 prior voluntary recalls of Metformin ER formulation tablets by different manufacturers.
As per FDA analysis, G901203 lot has higher levels of NMDA which is more than the Acceptable Daily Intake Limit. Lupin has not received any adverse event reports pertaining to this recall.
The product can be identified by the NDC and the lot number available on the side of the bottle label. Metformin Hydrochloride Extended-Release Tablets USP, 500 mg was distributed nationwide in the USA to wholesalers, distributors, and mail order pharmacies.
Discontinuation & Return of Recall Product:
Wholesalers, distributors, and retailers that have Metformin Hydrochloride ER Tablets USP, 500mg which is being recalled should immediately discontinue distribution of affected lot and to return the same to Inmar Rx Solutions, Inc., 635 Vine St, Winston Salem, NC 27101. Tel: (855) 532-1856.
For any queries regarding this recall:
Contact- Inmar Rx Solutions, Inc. at (855) 532-1856 Monday – Friday 09:00 am to 05:00 pm EST. For reimbursement, please have the recalled lot returned to Inmar Rx Solutions, Inc.; the lot number can be found on the side of the bottle.
Key Information for health care professionals/consumers:
- NDMA levels are not identified in Metformin immediate release (IR) formulations which are most commonly prescribed type of metformin medication compared to ER formulations. Also, NDMA is not identified in the samples of metformin active pharmaceutical ingredient
- If patients with type 2 diabetes stop taking the metformin prescribed by their physician, it could cause potential life-threatening risk to their health or Potential hospitalizations for hyperglycaemia and ketoacidosis. So, Patients should continue taking metformin tablets even after recall, until they consult with their respective health care professional who can prescribe an alternative medication.
- The FDA recommended health care professionals to continue prescribing metformin to diabetic patients when clinically appropriate.
Voluntary Side Effect Reporting to US-FDA:
Adverse reactions or quality problems experienced with the use of medicinal products may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report via MedWatch Online Voluntary Reporting
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178