European Medicines Agency’s (EMA’s) Pharmacovigilance Risk Assessment Committee (PRAC) recommended a test for identification of the “lack or inadequate presence of an enzyme, dihydropyrimidine dehydrogenase (DPD)” in the body before receiving treatment with fluorouracil (by injection or infusion) and the its prodrugs which included capecitabine and tegafur (these drugs are converted to fluorouracil in the body) and flucytosine (similar drug to fluorouracil). This recommendation is based on the safety review assessment which was initiated in March 2019 at the request of the French Food and Drug Administration (ANSM).
Fluorouracil (5-FU or 5-Fluoro Uracil) is an anti-cancer class of medication used in the treatment of various types of cancers (colon, oesophageal, stomach, pancreatic, breast and cervical cancer). Its topical formulation (cream) is used for treatment of actinic keratosis, basal cell carcinoma, and skin warts.
Capecitabine and tegafur are anti-cancer pro-drugs which are converted into the active drug, Fluorouracil after metabolism inside the body. Flucytosine is an anti-fungal medication which is similar in molecular structure to fluorouracil used in the treatment of severe fungal infections and some forms of meningitis (inflammation of the membranes that surround the brain and spinal cord).
Dihydropyrimidine dehydrogenase (DPD):
DPD is an enzyme present in body for the metabolism of fluoropyrimidines molecules. Fluorouracil, its prodrugs and flucytosine belongs to this fluoropyrimidines class and their catabolism is based on this enzyme levels. Catabolism means breakdown of molecule into smaller fragments which facilitates the elimination of these drugs from the body.
Patients taking these medications (fluorouracil, capecitabine, tegafur and flucytosine) having low levels of or complete lack of DPD enzyme in the body are prone to increased levels of this drug in the blood stream can result in severe and fatal toxicity. Higher amounts of fluorouracil can lead to neutropenia (low levels of neutrophils, a type of white blood cells needed to fight infection), neurotoxicity (damage to the body’s nervous system), severe diarrhoea and stomatitis (inflammation of the lining of the mouth). PRAC mentioned that “Up to 8% of the Caucasian population have low levels of a working DPD enzyme, and up to 0.5% completely lack the enzyme”.
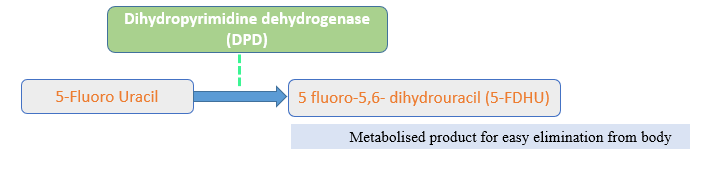
DPD level test:
It can be done by measuring the uracil level (a substance broken down by DPD) in the blood, or by checking for the presence of certain mutations (changes) in the gene for DPD which are associated with an increased risk of severe side effects.
- Patients with complete DPD enzyme deficiency: Patients should not be administered with these medications to prevent serious and life-threatening adverse events/reactions.
- Patients with a partial DPD enzyme deficiency: Patients should be prescribed with reduced dose of these medications followed by increased dose with regular monitoring of fluorouracil blood levels in patients.
PRAC stated that this DPD enzyme level test is not required for patients using topical fluorouracil, as the safety profile of topical fluorouracil is not associated with partial or complete DPD deficiency in patients. Because the level of fluorouracil absorbed through the skin into the body is extremely low to enter blood circulation.
DPD deficiency is a key factor for assessing benefit risk ratio for fluorouracil and its prodrugs. It was reported that 30-40 % of severe toxicities 60-60 % of treatment related toxicities are associated with diminished DPD activity. DPD deficiency is listed as an important identified risk in the risk management plan for capecitabine by its manufacturer.
Oncologists are advised to screen patients for DPD deficiency before initiating the treatment with fluorouracil and its prodrugs. Physicians are encouraged to screen patients before treatment with anti fungal medicine, flucytosine for DPD deficiency and to analyse the benefit risk ratio with respect to individual patient for safeguarding the patient’s health.