Food and Drug Administration (FDA) in the United States announced a serious safety concern on the use of hydroxychloroquine or chloroquine for the treatment of 2019 Coronavirus Disease (COVID-19) due to abnormal heart rhythms like QT prolongation and ventricular tachycardia.
Currently, there are no medicines proven effective for the treatment of COVID-19. On 28‑Mar‑2020, FDA released an Emergency Use Authorization (EUA), (authorization for temporary use during the COVID-19 pandemic for treatment of the hospitalized patients with COVID positive, when clinical trials are not available, or participation is not feasible) for hydroxychloroquine sulfate and chloroquine phosphate for the treatment of COVID-19. Based on limited evidence and anticipating the benefit, FDA authorized the use of these two medications for treating COVID‑19 patients only in hospitalized environment under careful heart monitoring by health care professionals. It was reported that increased use of the medicines has been observed even in outpatient prescriptions.
USFDA Approved Indications for Hydroxychloroquine sulfate and chloroquine phosphate: These two medications are approved for use in the treatment and prevention of malaria. Hydroxychloroquine is also approved for treating autoimmune conditions such as chronic discoid lupus erythematosus, systemic lupus erythematosus in adults, and rheumatoid arthritis. The benefit risk profile of these medication for use in COVID-19 patients is not yet evaluated. Clinical trial studies for these evaluations are still ongoing worldwide.
Safety profile of hydroxychloroquine and chloroquine:
The safety concerns well documented with use of these drugs include serious heart rhythm problems (QT prolongation and ventricular tachycardia), hypoglycaemia (low blood sugar) particularly among people with diabetes; anaemia, haemolysis in patients with Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency, worsening of seizures and other brain problems, retina (layer of eye tissue) damage that can cause vision problems.
These drugs can cause more serious problems when they are used in combination with other medications due to drug-drug interactions and potential impact on respective drug metabolising enzymes. The QT prolonging would be synergized when these drugs are administered with azithromycin and other antibacterial medications (having QT prolongation potential). Both hydroxychloroquine and chloroquine have prolonged half-lives, between 40 and 50 days, and low blood clearance (e.g. hydroxychloroquine’s blood clearance is 96 ml/min). (For more information on safety profile: USFDA Fact sheet)
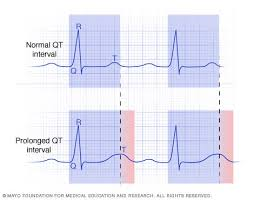
FDA’s Adverse Event Reporting System (FAERS) has received case reports of severe adverse events which included QT interval prolongation, ventricular tachycardia and ventricular fibrillation, and some death cases associated with hydroxychloroquine and chloroquine use in COVID-19 patients (both from hospital and outpatient settings). These reports were received from published medical literature and American Association of Poison Control Centers National Poison Data System.
Advice to patients:
- The benefit risk profile of hydroxychloroquine and chloroquine is well evaluated under recommended doses for the approved indications (listed above). Patients using these medications for approved indication can continue to use as per their prescription.
- Refrain buying these medications without a prescription from online pharmacies.
- Patients should share the information on past and concurrent medication details (including vitamins and herbal products) to their treating physicians for assessing the possibility of drug-drug interactions and other potential safety concerns. Also patient should share information on any drug allergies, heart condition (including heart rhythm problems), kidney disease, liver disease or hepatitis, diabetes or problems with low blood sugar, blood problems such as G6PD deficiency, an eye problem involving the retina, alcohol consumption etc.,
- To report any suspected adverse drug events or reactions to FDA by submitting a online MedWatch form for voluntary reporting.
Advice to Health care professionals:
To perform evaluation and monitoring of below recommended tests before and after initiating treatment with hydroxychloroquine and chloroquine.
- Baseline ECG (Monitoring throughout the treatment duration for any changes in QT interval)
- Electrolytes evaluation (To address haemolytic conditions in patients with G6PD deficiency)
- Renal function tests (Higher risk of QT prolongation in patients with renal insufficiency/failure.
- Liver function tests (Potential hepatic impairment with chloroquine)
- To assess possible drug interactions for reducing any potential risks to patients.
Health care providers are advised to report any suspected adverse drug events or reactions by submitting a online ADR reporting form voluntarily to strengthen evident data on safety profile of these medicines.