A new drug safety signal was triggered from the WHO’s global drug safety database for pharmacovigilance activities. This signal was identified by the UMC (Uppsala Monitoring Center), a WHO Collaborating Centre for international service and scientific research within the field of pharmacovigilance.
Agomelatine
Agomelatine is an atypical antidepressant medication used to treat major depressive disorder. This molecule is similar in structure to melatonin (a neurotransmitter produced by pineal gland in the brain) and acts as potent non-selective agonist for melatonin receptors, MT1 and MT2 and as an antagonist on neutral serotonergic 5-HT2C receptors, which is the key for its antidepressant effect and beneficial effect on sleep.
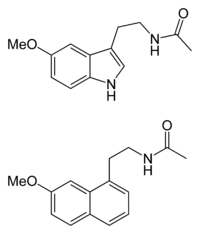
Melatonin and Agomelatine (Similarity in Molecular structures)
Global case reports in VigiBase
As per the recent newsletter (No. 2, 2020), released by WHO, there are total 24 case reports pertaining to “increased blood pressure” with “agomelatine” use in the VigiBase, (WHO global database of individual case safety reports). These reports were originated from eight different countries [Germany (15 case reports), Austria (2), Switzerland (2), and Australia, the Czech Republic, Portugal, South Africa, and Turkey (one each)] during the period Dec-2012 and Nov-2017. These cases were reported by both health care professionals (22) and non-health care professionals (2).
Out of these 24 reports, 12 case reports were found eligible for detailed analysis. Remaining 12 cases were excluded on basis on other contributor factors for the increased blood pressure, which included use of concomitant medications with ‘hypertension as a labelled adverse drug event’, underlying medical conditions (hypertension) and poor reporting of safety information in some cases.
Significant information from 12 case reports:
Six cases revealed a consistent pattern of a short time to onset (Reasonable time relationship between agomelatine administration and onset of increased blood pressure). Nine cases reported positive dechallenge (recovery after agomelatine withdrawal) and out of 9 cases, two cases showed a positive rechallenge (increased blood pressure upon re-administration of agomelatine). One case reported recovery after reduction of agomelatine dose. In addition to this, there are 17 case reports in the VigiBase database (found on 13-Oct-2019). These cases also have reasonable time relationship for the onset of adverse event symptoms after drug administration, positive dechallenge (4 cases) and with no concomitant medications (6 cases).
Agomelatine’s Hypotensive effect:
In contrast to the identification of above causal association between increased blood pressure and agomelatine, UMC published a signal in the year 2014 newsletter, for occurrence of hypotension with agomelatine treatment and Marketing Authorisation Holder (MAH) accepted the antihypertensive effect based on antagonistic effect of agomelatine on 5-HT2C/5-HT2B receptors and it was not endorsed as a clinically relevant safety concern. It is well-known that some medicines that usually lower blood pressure may paradoxically increase blood pressure. For example, pregabalin is a drug used for generalized anxiety disorders that have both “hypotension and hypertension” as labelled adverse drug reactions with the same frequency (unknown). The MAH’s statements in certain ICSR case narratives that the cases do not trigger any changes in the core data sheet are fully agreed upon, the reaction is far from being proven and can be extremely rare. As per the safety evidence availed from clinical trials, Agomelatine had neutral effect on heart rate and blood pressure.
The data on the mechanism of action of agomelatine (serotonin antagonism and melatonin agonism), as well as the former WHO’s VigiBase signal of 2014, suggests a mild hypotensive effect rather than hypertensive action. It is also true that melatonin has hypertension as a labelled adverse effect, which is closely related to agomelatine structurally. Thus, despite the presence of additional risk factors for hypertension in a considerable proportion of these cases, a contributory role of agomelatine to the events cannot be excluded.
In a wider perspective, agomelatine has the potential to alter the blood pressure (which could either increase or decrease blood pressure).